Thread
In all domains of life, natural selection crafted mechanisms to defend against pathogens. Remarkably, some of the evolved proteins are shared among all domains of life, including nucleotide binding leucine-rich repeat immune receptors (NLRs) 1/n
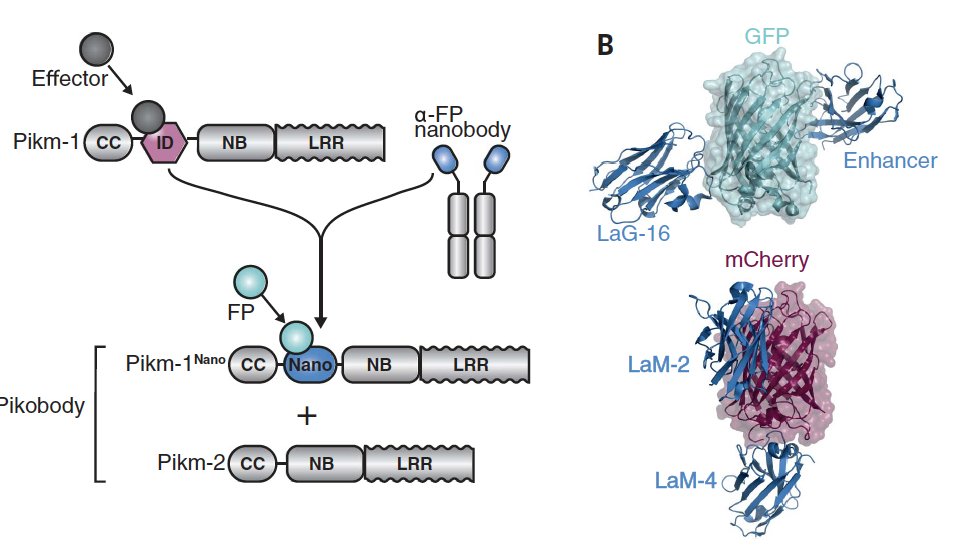
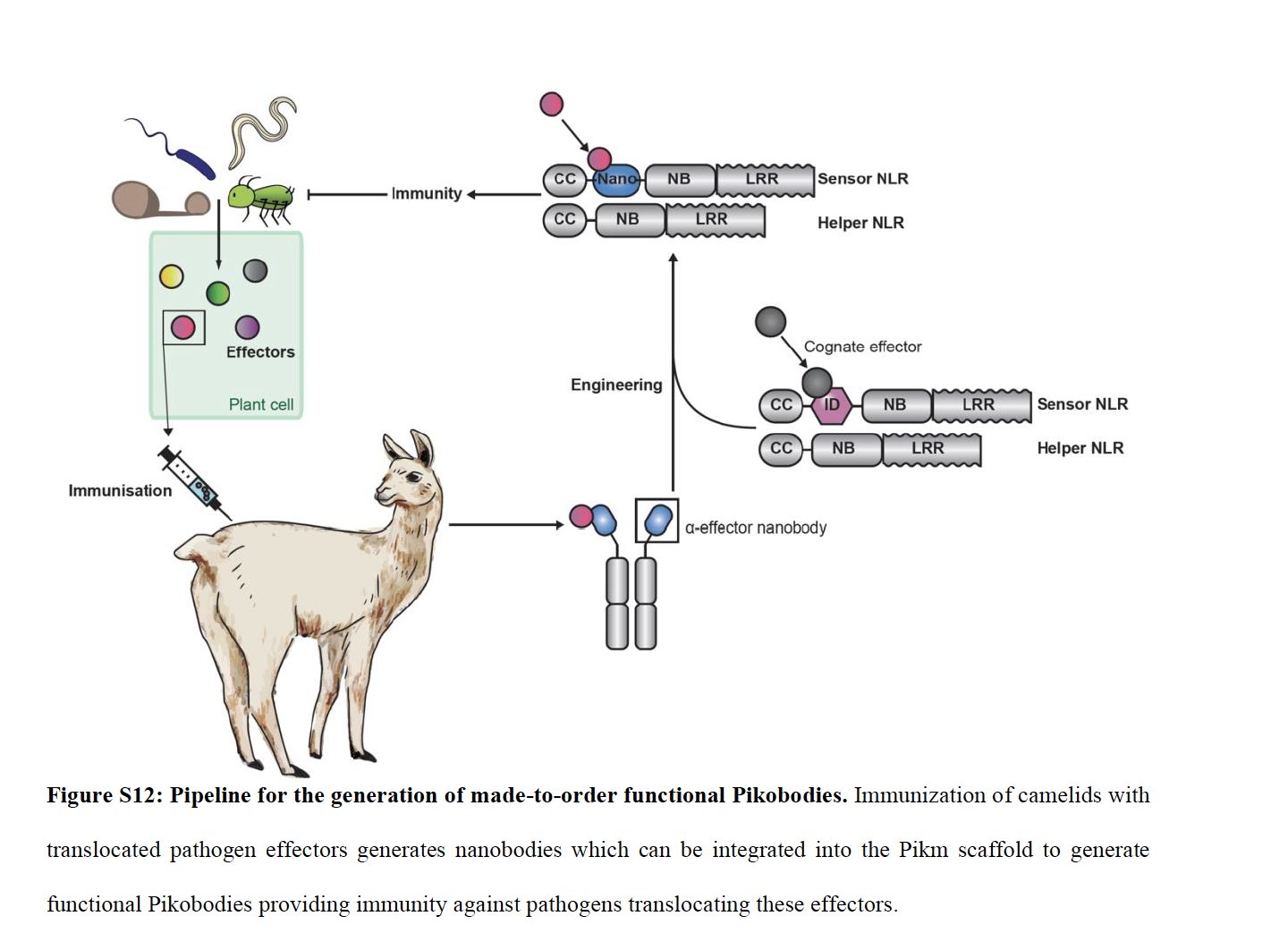
NLRs are key parts of the inate immune systems of animals, plants & bacteria, recognizing pathogen features- generalized like peptidoglycan or more specific like bacterial effector proteins. In some plant NLRs unconventional integrated domains are the recognition module 2/n
However, these receptors lack the broad adaptability of the vertebrate adaptive immune system, which can generate antibodies against virtually any antigen to which it is exposed. The @KamounLab now has developed a clever approach to bridge this gap 3/n
pubmed.ncbi.nlm.nih.gov/36862785/
pubmed.ncbi.nlm.nih.gov/36862785/
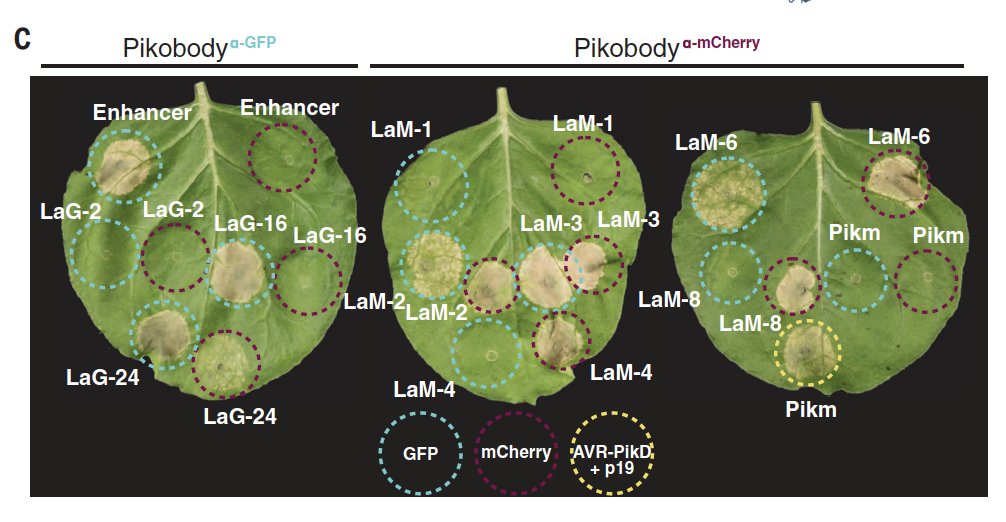
The approach is deceptive simple: replace the unconventional integrated domain with the recognition domain of a single chain antibody (a nanobody). As a proof of principle, they used well characterized nanobodies against GFP or mCherry 4/n
They tested an array of such nanobodies, looking for ones that triggered a protective hypersensitive response that was specific for the targeted antigen 5/n
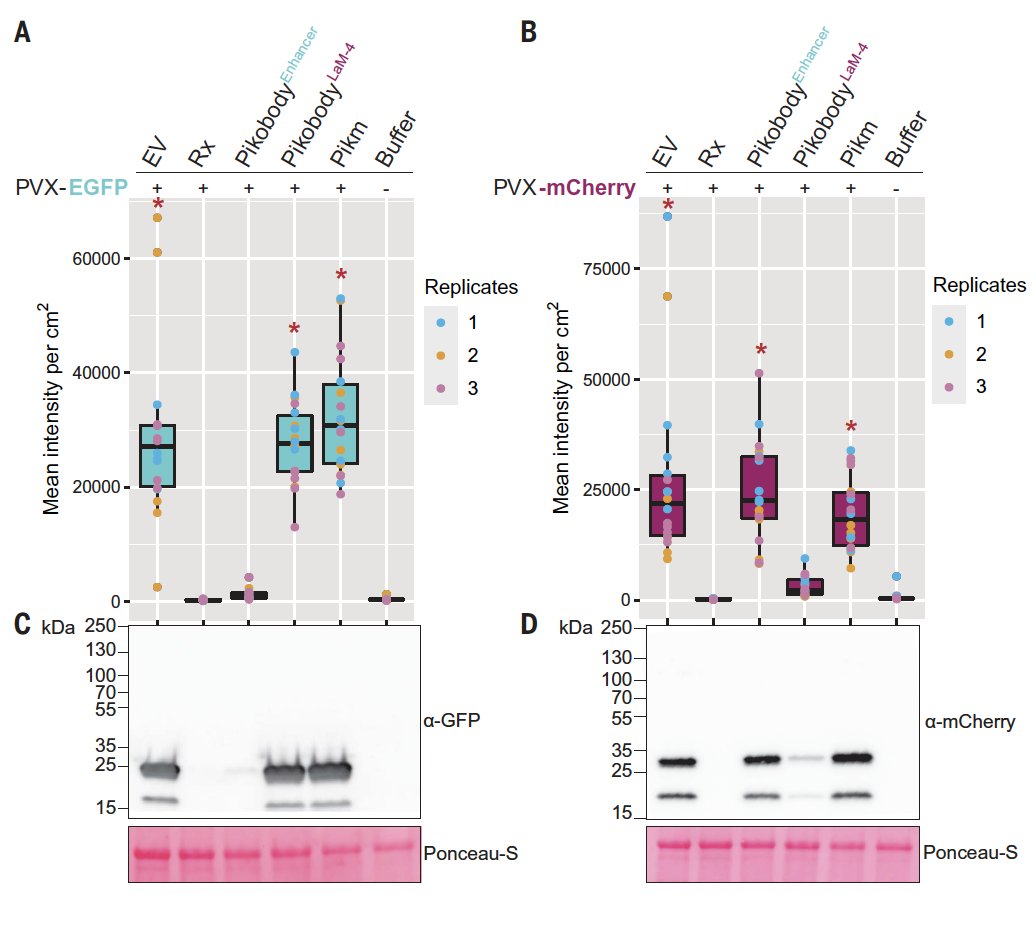
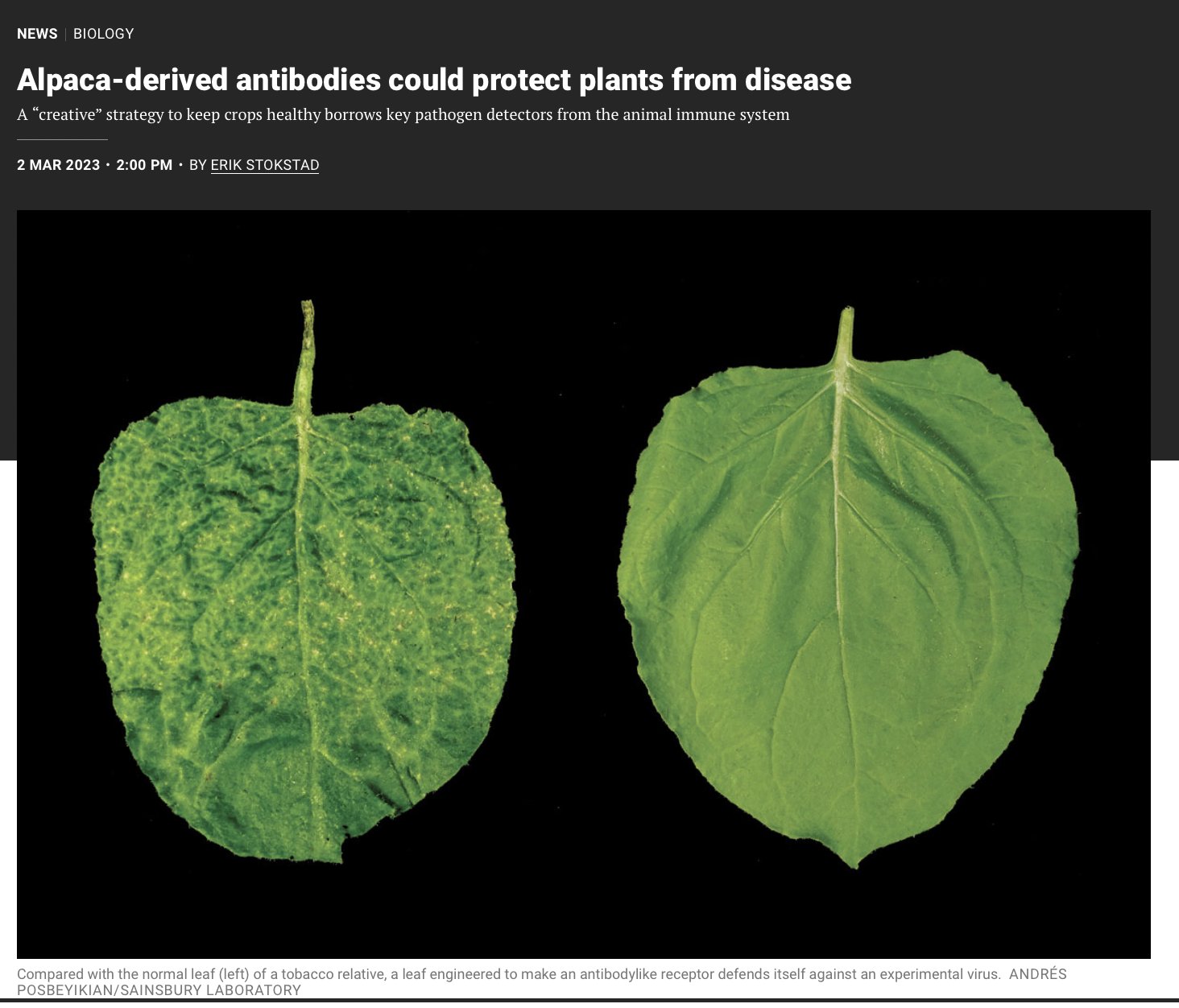
They next showed these engineered NLRs could reduce the titer of viruses engineered to express with GFP or mCherry 6/n
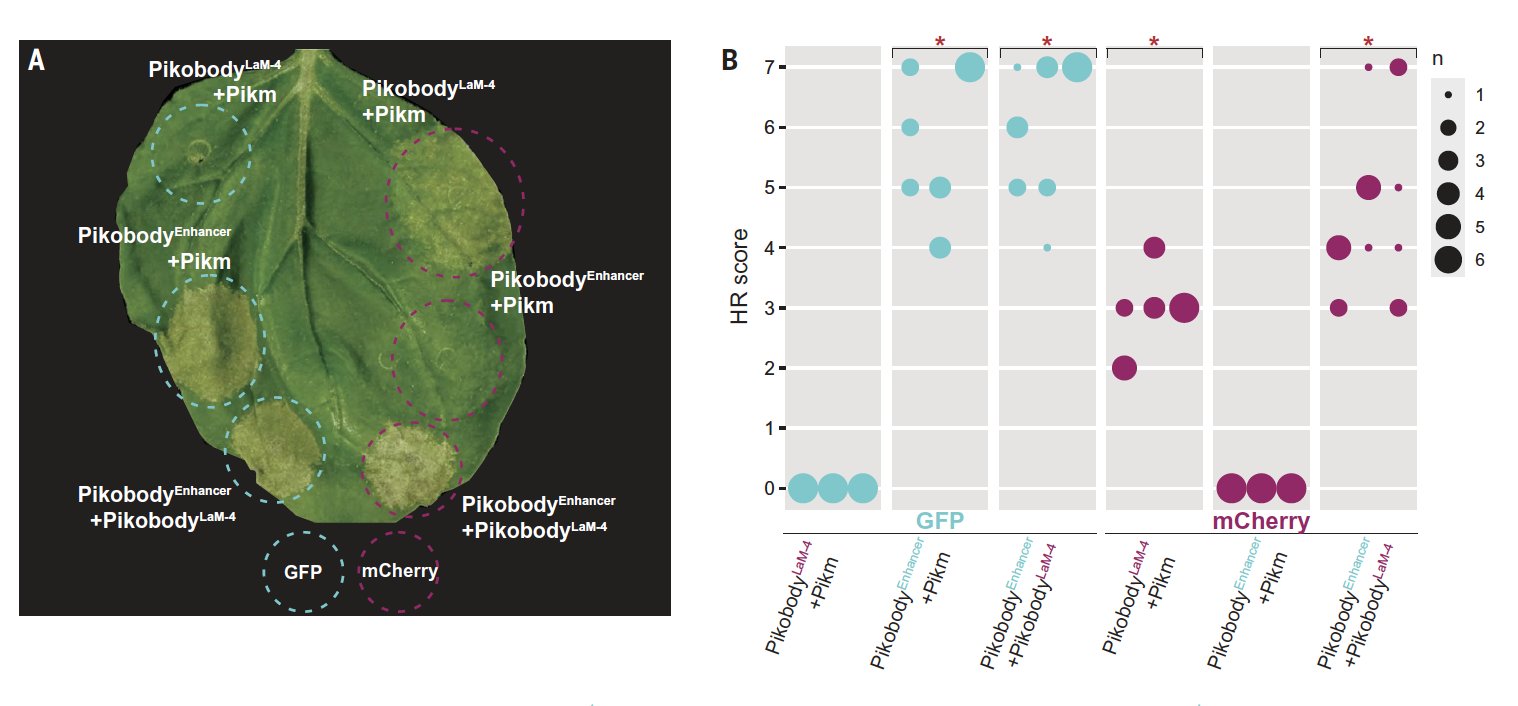
In the field, farmers would love to "stack" resistance genes, combining them in a single plant-- they found their engineered NLR proteins could act in this way, conveying dual resistance 7/n
As the authors note, "given that nanobodies can be
readily generated to bind virtually any antigen,
Pikobodies have the potential to produce
made-to-order resistance genes against any
pathogen or pest that delivers effectors inside
host plant cells" 8/n
readily generated to bind virtually any antigen,
Pikobodies have the potential to produce
made-to-order resistance genes against any
pathogen or pest that delivers effectors inside
host plant cells" 8/n